|
Bacterial leaching of ores and other materials R. Näveke, Institut
für Mikrobiologie, Technische Universität |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary
In nature sulfidic ores are decayed by weathering under the influence of oxygen and water. Microbiological investigations reveal that certain bacteria are the main agent in this process. Several bacteria, especially Thiobacilli, are able to solubilize heavy metal minerals by oxidizing ferrous to ferric iron as well as elemental sulfur, sulfide and other sulfur compounds to sulfate. So they enhance leaching of heavy metals from sulfidic ores under aerobic conditions about 104 fold or more compared with weathering without bacteria. The principal bacterium in ore leaching is Thiobacillus ferrooxidans, which is capable of oxidizing ferrous iron as well as sulfur and sulfur compounds. But there are some other bacteria which may also be involved. For example the thermophilic Sulfolobus plays a role in leaching at elevated temperatures. Thiobacillus thiooxidans, which oxidizes merely sulfur and sulfur compounds but not iron, and Leptospirillum ferrooxidans, which contrarily oxidizes only ferrous iron, may play a role if they work together or with other bacteria. Bacterial ore leaching can be applied to extract heavy metals from low grade ores, industrial wastes and other materials on an industrial scale by different procedures: dump leaching, in situ leaching, tank leaching, leaching in suspension. Sulfidic copper and uranium ores are the principle ores leached in several countries. So 20% to 25% of the copper production in the U.S.A. and about 5% of the world copper production is obtained by bacterial leaching. This process is a very slow one and needs a long time (years) for good recovery, but its main advantages are low investment costs and low operating costs. Current investigations deal with the leaching of ores other than those mentioned, leaching industrial wastes to recover metals, desulfurizing of coal, developing methods for in situ leaching and using other microorganisms than those used until now. Basic microbiological research focuses on the biochemistry, physiology and genetics of the involved microorganisms and on the complex interrelationships in the microbial community of leaching biotopes.
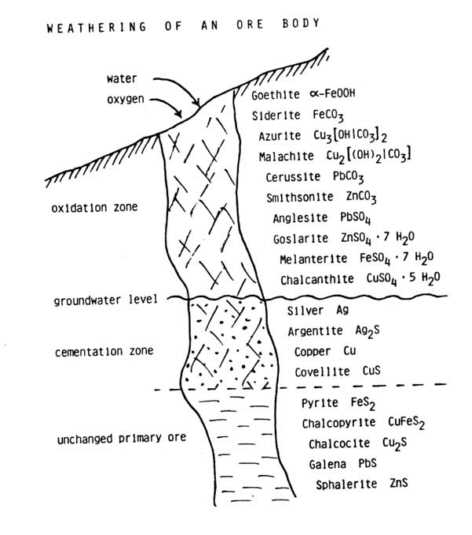
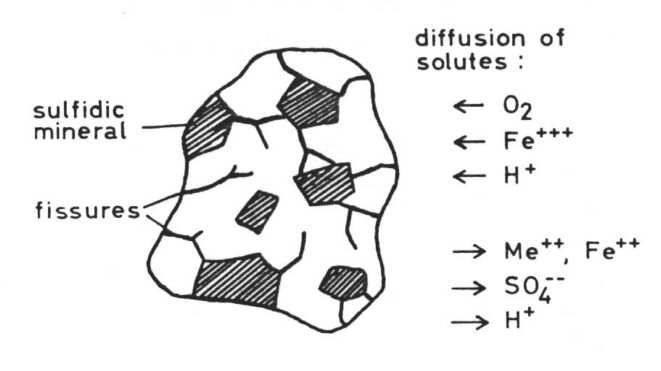
It is a fact that resources of metal ores are limited and that sooner or later these resources will be exhausted. But how great are our resources in naturally occurring deposits? Before we answer this question we have to define what a metal ore deposit is. A metal ore deposit is a naturally occurring concentration of a metal or some metals from which this metal can be obtained in an economic way. So, whether or not a deposit of metal ore can be considered are source or not depends on the costs we have to pay for extracting the metal from the ore and on the price we can get for the pure metal on the market. In other words: If the price of a metal rises -as is to be expected with depletion of the resources- and the costs of extraction are lowered, the amount of resources in the world rises. Microbial leaching of ores depends primarily on bacterial processes which are the essential causes of natural weathering of sulfidic minerals. If sulfidic heavy metal minerals come into contact with air and water they begin to decay with the formation of sulfate, sometimes sulfuric acid, and water soluble heavy metal cations. Weathering of an ore body results in a typical picture:
Microbiology of ore leaching Microbiological investigations
revealed that certain bacteria are the main agent in natural weathering
of sulfidic heavy metal minerals.
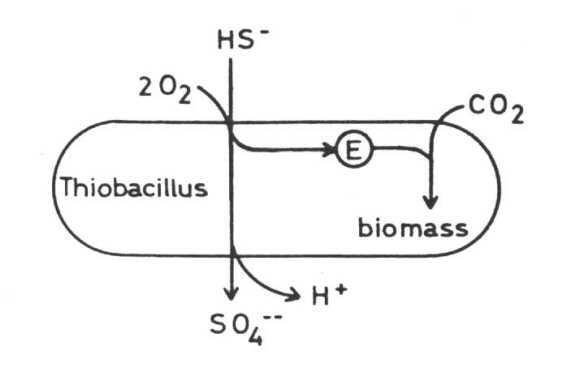
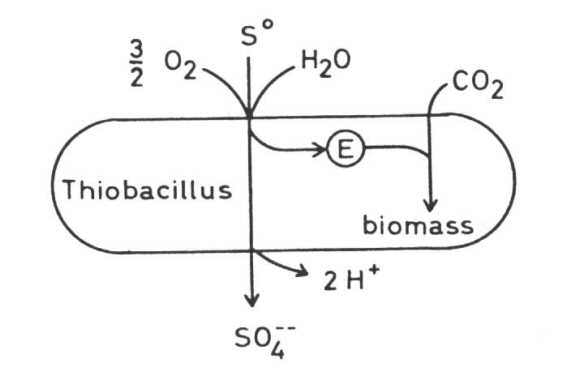
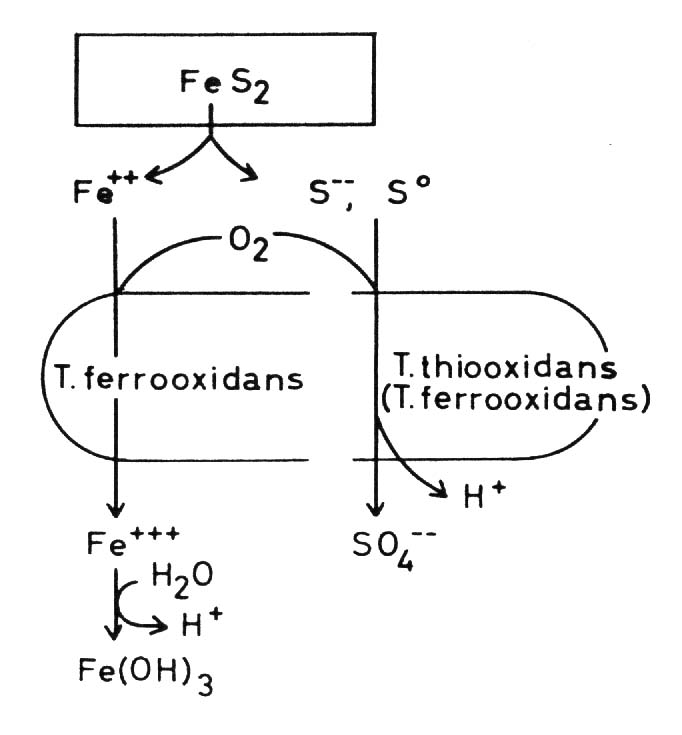
Thiobacilli The principal bacteria which play the most important role in solubilizing sulfidic metal minerals at moderate temperatures are species of the genus Thiobacillus. They are gramnegative rods, either polarly or nonflagellated. Most species are acidotolerant, some even extremely acidotolerant and acidophilic. Some grow best at pH 2 and may grow at pH 1 or even at pH 0.5. Most species are tolerant against heavy metal toxicity. Thiobacilli are chemolithoautotrophs, that means CO2 may be the only source of carbon and they derive their energy from a chemical transformation of inorganic matter. All Thiobacilli oxidize sulfur or sulfur compounds to sulfate or sulfuric acid.
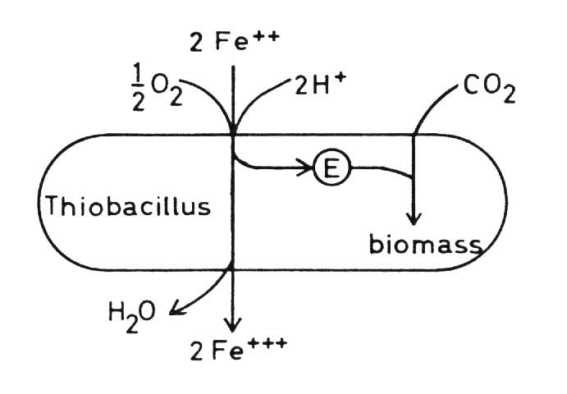
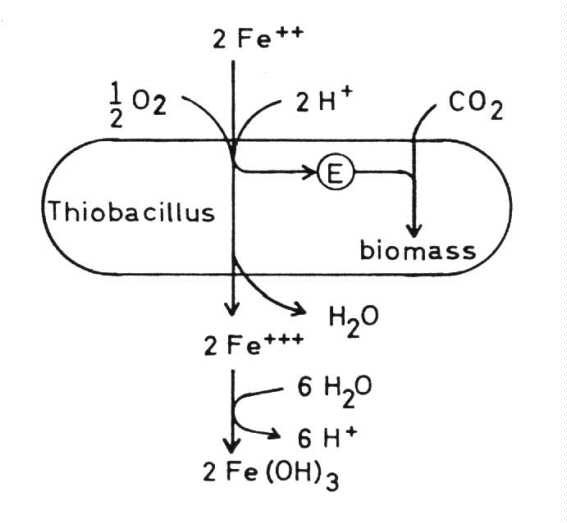
Thiobacillus ferrooxidans In addition to the oxidation
of sulfur and sulfur compounds Thiobacillus ferrooxidans is able to oxidize
ferrous to ferric iron and so derive its energy from this exergonic reaction.
In this reaction hydrogen ions are consumed and so the pH of the medium
should rise. But at pH values higher than 2 the ferric iron precipitates
as ferric hydroxide, jarosites or similar compounds and this results in
the formation of hydrogen ions, so that the pH of the medium is lowered
as is the case with oxidation of sulfur compounds:
As will be shown later,
owing to its ability to oxidize ferrous iron, T. ferrooxidans is
the principal agent of bacterial ore leaching at moderate temperatures.
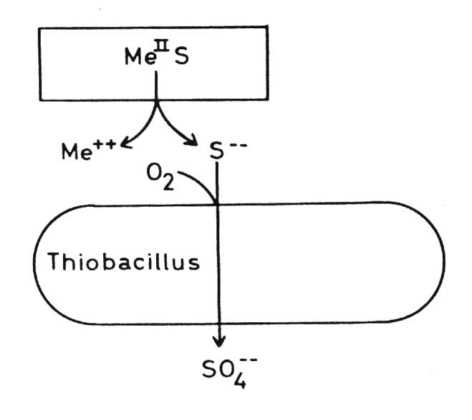
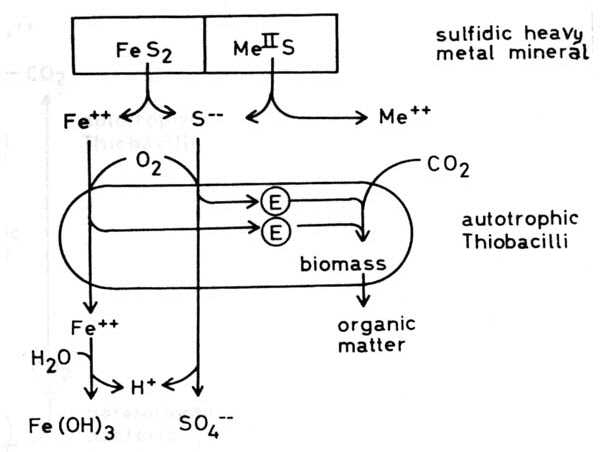
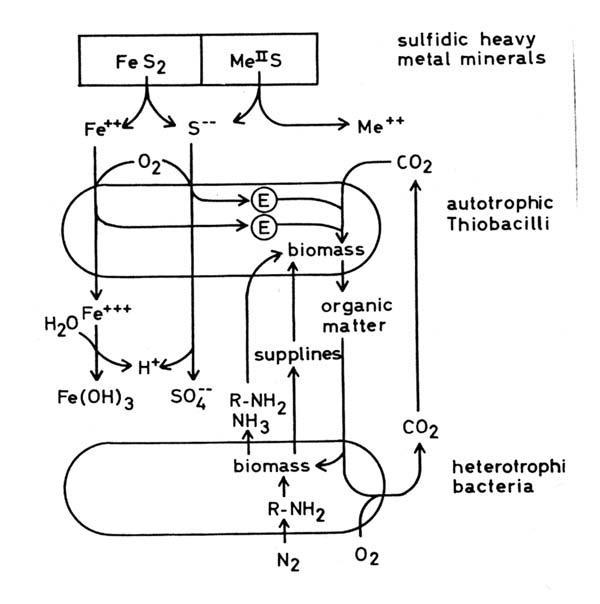
Thiobacilli and sulfidic minerals Some Thiobacilli, especially T. ferrooxidans, are able to oxidize sulfide and some heavy metals -mainly iron but also copper, zinc, molybdenum and presumable some other metals - in the form of sulfidic heavy metal minerals which are of very low solubility in water, practically insoluble. These oxidations result in a solubilization of the minerals. This is often seen in the case of pyrite or marcasite, both FeS2, minerals which are oxidized very easily by Thiobacilli: but also in the case of other minerals. Oxidation of the sulfide of a divalent metal:
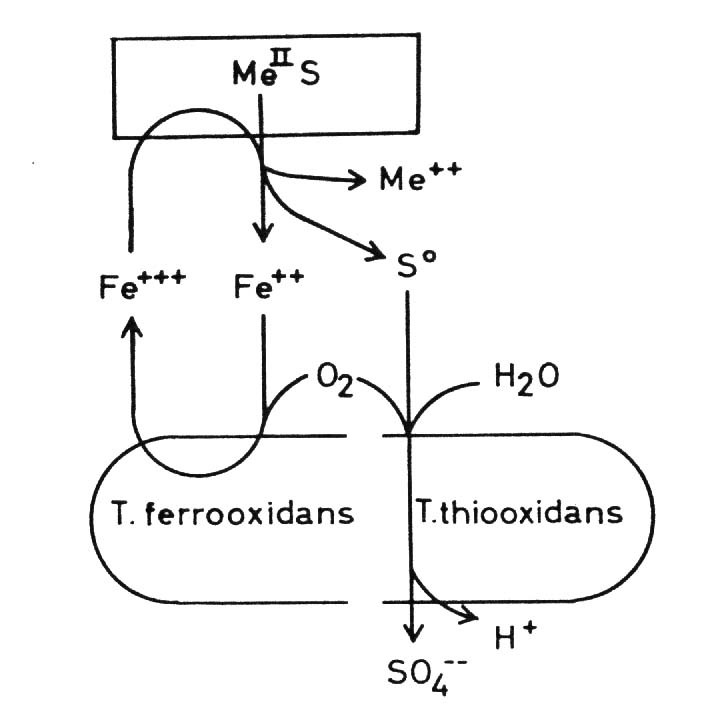
(c) The combination of hydrogen ion attack and oxidation with oxygen releases metal ions and elemental sulfur: in the case of chalcocite (Cu2S) it forms covellite (CuS), and copper ions: These processes are called the direct mechanisms of bacterial mineral solubilization to distinguish them from an indirect mechanism: (d) Ferric ions, - formed by oxidation of ferrous iron by T. ferrooxidans, are a strong oxidant and may oxidize sulfidic bound metals so that soluble metal cations are formed: The elemental sulfur may be oxidized by Thiobacilli to sulfuric acid which supports the dissolution of the mineral according to equations (8) to (11):
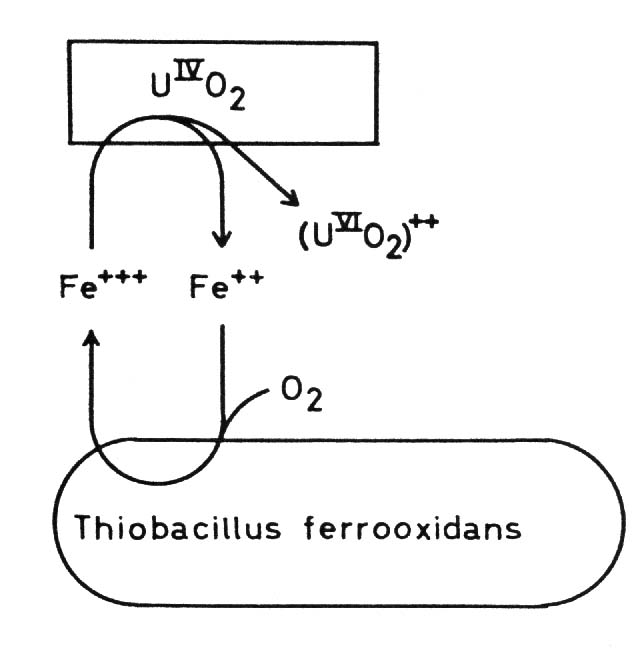
By this indirect mechanism of bacterial dissolution of sulfidic minerals also heavy metal minerals can be attacked which are not accessible to the direct mechanisms, especially whose metals which can not be oxidized by the bacteria. Moreover some non-sulfidic heavy metal minerals can be brought into solution through oxidation mediated by the ferric/ferrous iron system. This latter fact is of particular importance in leaching uranium ores: uranium(IV) for example as uranium dioxide UO2, uraninite, is oxidized by ferric iron to uranium(VI) and so soluble uranyl ions UO2 are formed: Thiobacillus thiooxidans, an
extremely acidophilic but not ferrous iron oxidizing species of the Thiobacilli,
is not able to solubilize sulfidic heavy metal minerals in pure culture.
Nevertheless T. thiooxidans plays a role in metal leaching. The solubilization
of sulfidic minerals by Thiobacillus ferrooxidans is increased by cooperation
with T. thiooxidans as compared with the effect of T. ferrooxidans alone.
We can assume that the cause of this enhancement is the oxidation of elemental
sulfur and hydrogen sulfide which is formed as a result of the oxidation
by ferric iron according to equation (12), for this oxidation produces
hydrogen ions which in turn attack the minerals according to equations
(8) and (9).
Other bacteria In addition to Thiobacilli there are some other bacteria known to be effective in solubilizing sulfidic minerals. In hot biotopes containing sulfur or oxidisable sulfur compounds, such as hydrothermal vents and self heating brown coal dumps, one can find an archaebacterium named Sulfolobus. This is a bacterium without a rigid cell wall, round shaped, about 0.8 to 1.0 §m in diameter. Like Thiobacilli it is acidophilic, chemolithoautotroph and derives its energy from oxidation of sulfur and sulfur compounds and from oxidation of ferrous iron like Thiobacillus ferrooxidans. Its pH-range of growth is pH 1.0 - 6.0 and its optimum at about pH 2. A salient characteristic is its thermophily: its growth range is 45 – 85°C, its optimum 70 – 75°C. Species of this genus, especially S. brierleyi seem to be the main agent in metal leaching at high temperatures.
Leptospirillum Often one can see in acid metal leaching biotopes spirilloid bacteria. They belong to the species Leptospirillum ferrooxidans, a gramnegative spirillum, facultatively chemolithoautotroph, deriving its energy from oxidizing ferrous iron like Thiobacillus ferrooxidans. But in contrast to this latter bacterium it cannot oxidize sulfur or sulfur compounds and is incapable of utilizing the iron of sulfidic minerals. Leptospirillum ferrooxidans alone
cannot solubilize sulfidic ferrous iron containing minerals. But in cooperation
with Thiobacillus thiooxidans, which, for its part alone, is also unable
to dissolve sulfidic minerals, it can; both bacteria together disintegrate
sulfidic ferrous iron containing minerals by oxidation and bringing them
into solution (Balashova et al., 1974).
Bacterial leaching versus abiotic leaching Simple laboratory experiments
can show, that chemical reactions catalyzed by bacteria are the essential
processes which lead to decay of sulfidic heavy metal minerals and some
other minerals and that abiotic reactions play a negligible role. If sulfidic
ores are percolated with simple water or diluted salt solutions under aeration
in laboratory percolators in parallel sets, one set not sterilized or inoculated
with natural acid mine effluent, another set under sterile conditions,
it can be seen that disintegration of ore and leaching of metals proceeds
in the not sterilized or inoculated percolators very much quicker than
in the sterilized ones, the ratio being about 104 or higher.
Bacterial disintegration of ores has been applied on a technical scale for many years, almost solely to leach copper and uranium. Actually it was used for extracting copper from sulfidic ores long ago and long before bacteria were recognized as the cause of natural weathering. In some places ore leaching was operated some centuries ago, for instance at Rio Tinto in Spain. In the last decades bacterial ore leaching was carried out in many countries: Canada, U.S.A., Mexico, Australia, India, U.S.S.R., Turkey, Yugoslavia, Romania, Hungary, Spain and some other countries.
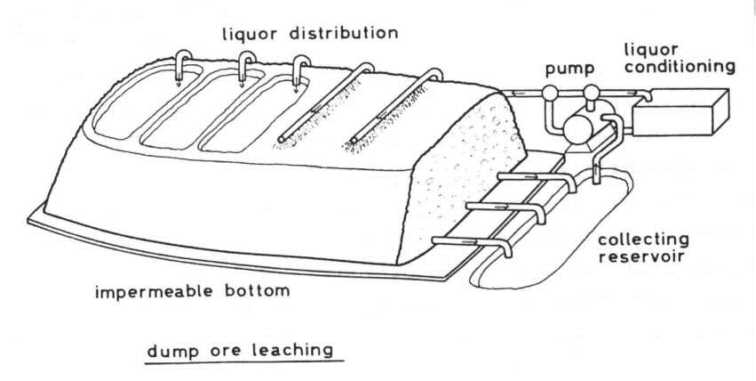
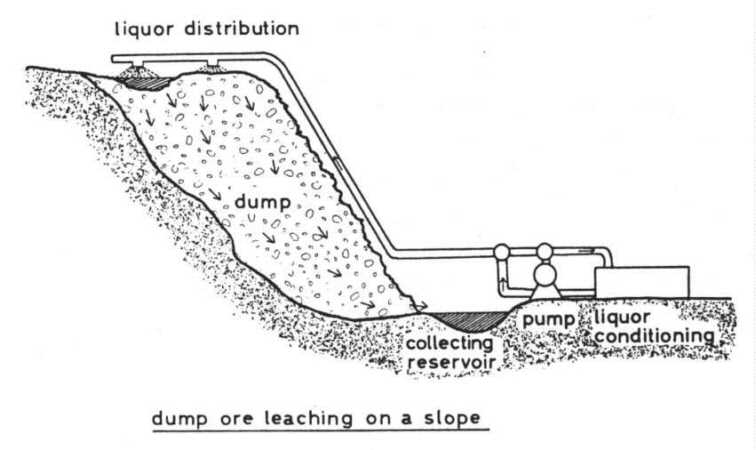
Dump leaching The most commonly applied method is that of the percolator principle. Big dumps of ore are set up on an impermeable ground. The grain size has to be so that on the one hand the leaching liquor can percolate through the dump and air may enter from the sides, and on the other hand the distances for mass diffusion inside the grains are as short as possible. The leaching liquor is distributed
on the top of the dumps by sprinklers or by intermittent flooding of ponds.
At the bottom the liquor is collected, in some cases by a drainage system,
and conducted to a collecting reservoir from which it is pumped back on
top of the dump. Before pumping back to the dump the whole liquor or a
part of it may be conditioned, that means extracting the dissolved metal
(for instance copper by cementation with iron scrap), addition of sulfuric
acid if the pH is too high and addition of nutrient salts if desired.
The pH of the circulating liquor is about 2.0 - 3.5, its iron concentration about 35 - 60 mmol/l. In the on-flowing liquor the iron is almost completely ferrous iron, whereas in the outflow only 108 to 40%, sometimes 70%, of the iron is ferrous iron. So we can conclude, that iron is oxidized by the bacteria almost exclusively inside the dump. This fits with the observation, that almost all bacteria adhere on the ore and only a small amount is free in the fluid as mentioned above. Therefore a good aeration of the dumps is necessary, but this occurs unaided at least in their outer and upper parts by thermic air buoyancy for the temperatures in the dumps are elevated by the reaction heat up to 30 – 40°C, and in some spots temperatures near 60¡C were measured. By the way: out-streaming air at the top of, the dumps contains much less oxygen than does normal air. In most cases the addition of nutrients is not necessary because Thiobacilli are lithoautotrophs and need only some inorganic nutrients besides an energy source. The required inorganic nutrients may be taken from the ore. The nitrogen source may be an exception for ores usually contain only small amounts of nitrogen compounds. But it has been found that strains of Thiobacillus ferrooxidans are able to reduce molecular nitrogen and so meet their demand for nitrogen (Mackintosh, 1978). Operating big dumps the circulation rate is about 5000 m3 of liquor per hour (20 -30 l × m-2 × h-1). The copper concentration of the out-flowing liquor is about 8 mmol/l (500 g/m3). In the U.S.A. 200,000 to 250,000 t of copper are produced annually by bacterial leaching, equivalent to 20 -25% of the total copper production. In the whole world about 5% of the total copper production is obtained by bacterial leaching. Bacterial leaching is a very slow process. Around 3 to 10% of the copper content is leached out of a low grade sulfidic copper ore per year. So dumps may be operated 10 to 20 years. But on the other hand dump leaching is a simple and cheap method. It needs only a little capital investment, has low operating costs, requiring-little labor, and is well-suited to low grade ores if they contain the metal in sulfidic minerals or if sulfides are contained in addition. A certain amount of pyrite in the ore is favourable because oxidation of pyrite by Thiobacilli releases enough hydrogen ions to lower the pH value and enough ferric iron for the indirect oxidation mechanism. Besides copper uranium is leached by bacteria from its ores on a technical scale. This leaching depends wholly on indirect oxidation by means of the ferric/ferrous iron system according to equation (15). So the leaching of uranium ores which contain pyrite as an iron source is most economical. Otherwise one has to add pyrite or another source of iron. The technical set-up of uranium ore leaching may be the dump method, but sometimes a variation of this, so-called heap or basin leaching is applied. The ore is set up in basins. The mode of operation is preferably a two stage leaching: the out-flowing liquor, in which the iron is largely in the ferrous form, is treated in an oxidation pond. In this the liquor is aerated to enable Thiobacillus ferrooxidans to oxidize ferrous iron and to obtain the ferric iron required for oxidation of uranium! The oxidized liquor is then pumped back to the dump or basin.
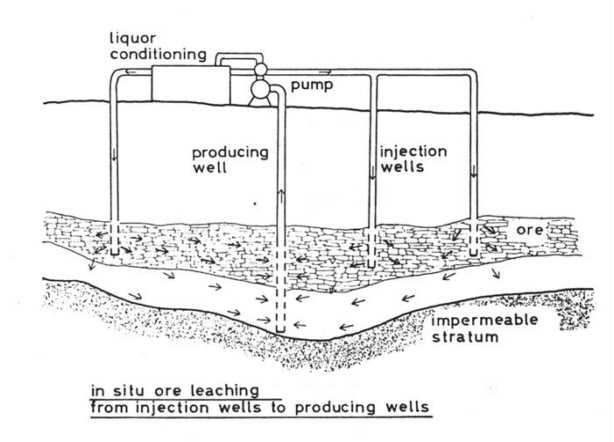
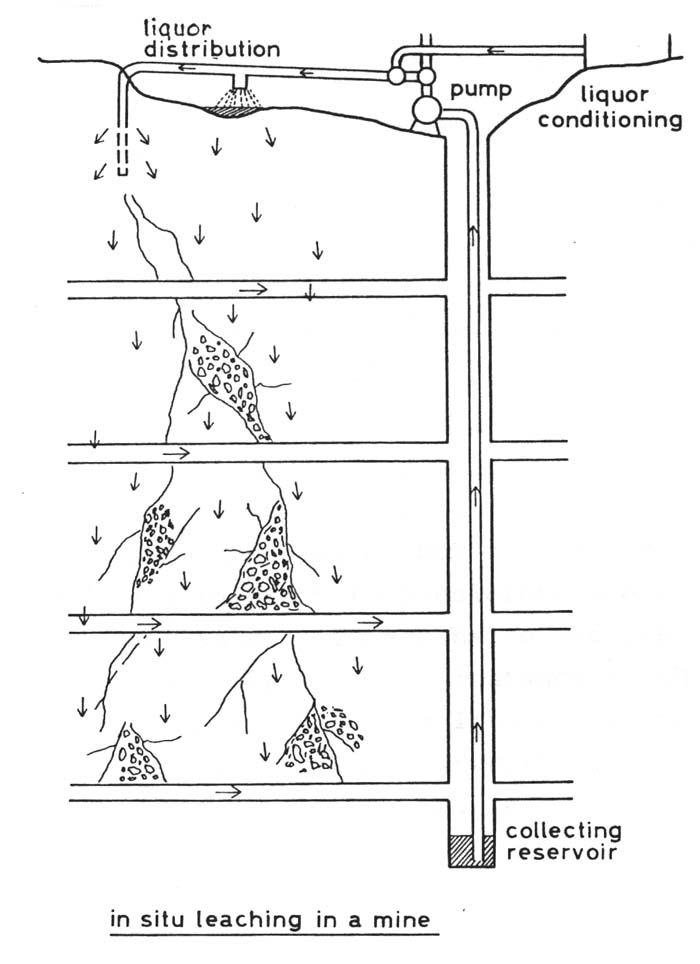
In situ leaching In a few cases it has been attempted
to leach ores by means of bacteria without excavating the ore prior to
leaching. At first sight it seems advantageously to leach ores on the spot
were they are, for excavating costs can be saved. But difficulties arise
if the ore body is impermeable or if there are only a few channels through
which the leaching liquor would stream downwards without percolating the
ore body entirely. In such cases the ore body has to be cracked by explosions.
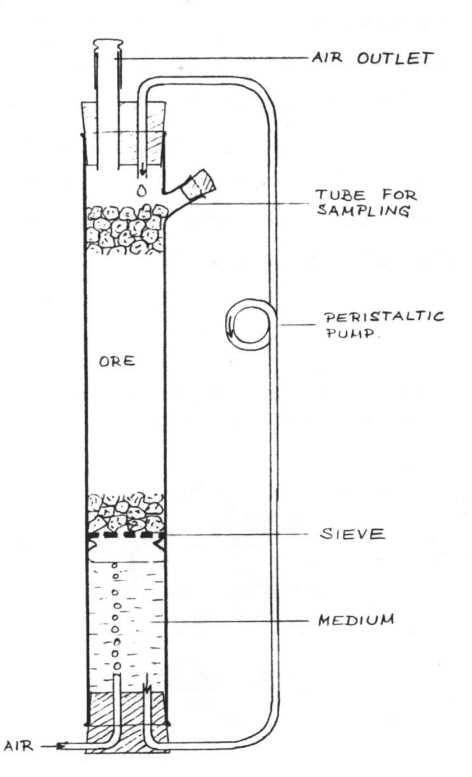
Other types of bacterial leaching plants Some other types of bacterial ore leaching arrangements were set up on a laboratory scale as well as on a semi-technical scale. Big tanks may be filled with pieces of ore like a laboratory percolator and then the ore may be percolated. Such an pilot plant has been set up at the John D. Sullivan Centre for In-Situ Mining Research in Socorro, New Mexico. The advantage of such a tank leaching is that the process can be easily controlled and regulated. The ore can be heated simply by insulating the walls of the tank, so the reaction heat of the oxidation is used for heating. Of course leaching in tanks is more expensive than dump leaching and could therefore be applied only to special purposes. A very interesting method is leaching ground ore in suspension. Grinding ore down to particle size of below 0.1 mm increases considerably the specific surface area and so increases the leaching rate substantially. But ore which is ground to low particle size cannot be percolated, it has to be treated in suspension. Therefore a reactor is required in which the suspension can be agitated and aerated. The pulp may contain 10% to 20% solids in suspension ("pulp density"). Suspension leaching is a very effective method and has the advantage that it can easily be controlled and regulated. So it may be possible to chose a favourable temperature and to add phosphate, ammonia, carbon dioxide, sulfuric acid, iron or other additives in order to accelerate the leaching process. But on the other hand it is expensive and its application is restricted to special purposes, for instance to the leaching of concentrates Suspension leaching on a laboratory scale in agitated flasks is a convenient tool to investigate the leachability of an ore and to reveal the optimal leaching conditions.
Problems There are many possibilities for disturbing bacterial leaching. Lack of iron can be met in most cases by adding iron in some form, preferably as pyrite because by oxidation of pyrite not only iron ions are formed but also hydrogen ions. Therefore addition of pyrite is well suited if it is necessary to lower the pH. For this latter purpose also elemental sulfur may be added instead of pyrite, Thiobacilli will then oxidize sulfur to sulfuric acid. A large amount of carbonates may cause serious disruption because Thiobacilli and other bacteria concerned with ore leaching are acidophilic. They are inactive and don't grow in a neutral or alkaline milieu. If enough hydrogen ions are formed by bacterial oxidation activity alkali of earth carbonates may be neutralized and decomposed. But then another problem arises: the alkali of earth ions precipitate as sulfates and these may disturb the leaching by plugging and by covering the surfaces of the ore minerals. In ponds on the top of dumps operated by the pond system ferric iron compounds often precipitate. This hinders the infiltration of the liquor by plugging the upper layer of the ore dump. From time to time the precipitates have to be scraped off. Toxic substances in the ore may
inhibit or kill the bacteria. Thiobacilli, Sulfolobus and
Leptospirillum ferrooxidans are very tolerant against dissolved
heavy metals. The following limits of heavy metal tolerance of T. ferrooxidans
were observed:
Arsenic, molybdenum, silver and mercury may be toxic to Thiobacilli. Noteworthy is the higher tolerance against molybdenum of Sulfolobus brierleyi: this bacterium metabolizes without inhibition at a molybdenum concentration of 20 mmol/l or higher, whereas Thiobacilli tolerate molybdenum only up to about 1 mmol/l (Brierley, Murr, 1973). In some cases the tolerance of leaching bacteria against toxic substances may be developed by adaptation.
Further investigations Many factors influence bacterial ore leaching: properties of the microorganisms mineral species including accompanying minerals surface area of the minerals, particle size water availability temperature pH redox potential oxygen supply carbon dioxide supply, supply of other nutrients e.g. nitrogen compounds, phosphate toxic substances light formation of secondary minerals Much work has been done on the influence of these factors, qualitatively and quantitatively. Further effort is needed to understand fully all dependencies in all cases of bacterial leaching. Much work has to be done in order to find new applications and new methods. Many research groups in several countries work in this field. An interesting approach is genetical manipulation of leaching bacteria. But here I should confine myself to report what is done in the Federal Republic of Germany. (a) Dr. Bosecker in his laboratory at the Bundesanstalt für Geowissenschaften in Hanover investigates the application of bacterial leaching to new ores. In particular he has tried to leach copper from copper bearing black shale, nickel out of gabbro and other basic plutonic rocks, and zinc out of old dumps which were left by miners some centuries ago in Germany. (b) Bacterial leaching of industrial waste materials is done on an laboratory scale and in pilot plants by the group of Prof. Onken in Dortmund and the German mining company Preussag at the Harz Mountains (Goslar). Tailings from flotation plants, metal containing drosses and similar materials are leached, mainly as suspensions in different bioreactors. (c) Coal often contains considerable amounts of pyrite which on combustion is oxidized to sulfur dioxide. To minimize the emission of this toxic and acid forming gas Dr. Ebner and his co-workers in a laboratory of the Bergbau-Forschung in Essen try to desulfurize coal by bacterial leaching. (d) As was mentioned above in situ leaching is problematic. The mining company Preussag set up an underground pilot plant in ah old mine in which a complex sulfidic ore has been excavated for more than a thousand years. In about 2 years it will be worked-out. In the upper and older parts of the mine the miners of past centuries left a lot of ore which is now slowly leached by natural bacterial oxidation and which cannot be excavated economically. The company is investigating whether or not these residues can be recovered economically by bacterial leaching. (e) Prof. Stetter and his group at the University of Regensburg have isolated about 350 new strains of bacteria, some of these, from hot springs and other hot biotopes in areas with volcanic activity in Italy and Iceland, are thermophilic, some extremely so. Prof. Stetter and his co-workers have examined these new strains in respect of their ability to leach metals out of ore minerals. Some 170 out of the 350 isolates are active in metal leaching. It may be that some of the thermophilic strains are suited to leaching at high temperatures with high rates on a technical scale. (f) The mining company "Uranerzbergbau" did some work on leaching special uranium ores and investigated conditions for leaching with rates which make this operation economical. (g) In our laboratory in Braunschweig we investigate the ecology of the microbial communities which develop in acid ore leaching biotopes. The main object of our work is an old mine in the Harz Mountains in which the mining company has installed an underground in situ leaching pilot plant. We have isolated a lot of microorganisms from this mine biotope in which the pH is between 2 and 3. We have been able to show that many neutrophilic, heterotrophic bacteria, isolated from the mine, become acidotolerant growing in a spent culture medium of Thiobacilli. This fact explains why so many neutrophilic bacteria are found in acid leaching biotopes. We wondered why so many heterotrophic bacteria live in an inorganic biotope which is thought to be free of organic matter, for heterotrophic bacteria need organic matter as an energy source and nutrient. The answer is that the autotrophic Thiobacilli are primary producers of organic matter, because they can synthesize biomass from carbon dioxide. We found that many of the heterotrophic bacteria isolated from this biotope can grow at the expense of organic matter produced and partly excreted by the Thiobacilli.
February 1986 |