| The microbial oxidation of ferrous
iron and particularly the claim of autotrophic growth on iron have been
subjects of controversy for many years (Molisch, 1910; Winogradsky, 1922;
Cholodny, 1926; Baas Becking and Parks, 1927; Cataldi, 1939; Starkey, 1945;
Pringsheim, 1949a,b). In neutral or alkaline waters a nonbiological atmospheric
oxidation of ferrous iron takes place. This has made it impossible to determine
the relative importance of microbial and purely chemical oxidations of
iron. Atmospheric oxidation would cease to be a factor in acid media, but
the classical iron bacteria do not develop under strongly acid conditions.
An environment rich in ferrous
iron and highly acid is found in the mine drainage water of some of the
major bituminous coal sections of the United States. Colmer and Hinkle
(1947) showed that the ferrous iron oxidation occurring in this acid mine
water was biological. The isolation of the organism responsible and certain
of its morphological and cultural characteristics have been described (Colmer,
Temple, and Hinkle, 1949). The present communication presents definitive
evidence that the bacterium is an autotrophic iron oxidizer.
Media, inoculation, and culture maintenance. The original medium consisted of FeSO4-7H20 to give 2,000 ppm ferrous iron, 0.1 per cent MgS04 7H20, 0.05 per cent (NH4)2SO4, distilled water, and enough conc H2SO4 to give a final pH of 2.0 to 2.5. More recently an iron level of 26,000 ppm ferrous iron has been adopted. Sterilization of the medium may be accomplished by autoclaving, or by filtering with a Seitz, sintered glass, or porcelain type equipment. Autoclaving gives a voluminous precipitate of ferric hydrate, but the loss of ferrous iron is actually negligible. To rid the medium of the ferric hydrate the precipitate may be allowed to settle and the supernatant solution decanted, or the solution may be autoclaved in a large short-stemmed separatory funnel and the ferric hydrate drawn off at the bottom. Using the separatory funnel, the removal of 50 ml of solution from 1,050 ml gets rid of the entire precipitate. Nitrate media were prepared by substituting 0.05 per cent NaNO, for the ammonium sulfate. Agar plates containing 26,000 ppm ferrous iron were prepared by using the basal medium with the addition of 2 per cent agar. To prevent hydrolysis of agar during sterilization, the ferrous sulfate was autoclaved separately in one third of the water; the other ingredients were autoclaved in the remainder of the water, and the two solutions were mixed just prior to plate pouring. With this much ferrous sulfate no H2SO4 was needed to give the desired pH. Growth was satisfactory in Erlenmeyer flasks or bottles but slow in tubes. The use of a rotary shaker or of air bubblers gave faster growth. The culture was maintained by inoculating liquid media with one ml of a culture that had effected nearly complete oxidation of ferrous iron in the solution medium. Transfers were made at approximately two week intervals. Experlmelltal flasks were inoculated with varying amounts of cultures developing in the solution medium. Analytical methods. Ferrous iron was determined by the Zimmermann-Reinhardt(Treadwell and Hall, 1942) volumetric titration with permanganate. Determinations of ferric and total iron were abandoned when it was found that the value for ferrous iron gave an accurate picture of the changes in iron content. Carbon analyses were conducted by the Van Slyke-Folch (1940) procedure for wet combustion. The 2 ml samples used were dried overnight in a vacuum desiccator. Growth on synthetic iron media. The bacterium was kept on the original synthetic medium for 24 months by subculturing every two weeks, but it has been noted that the culture remains viable in closed bottles stored for several months. Growth was faster on the agar or solution media containing 26,000 ppm ferrous iron, being appreciable in 3 to 5 days. Motility was most marked on young agar streaks. At the higher iron concentration the bacteria were vastly more numerous as shown by microscopic observation. No growth was detected when the content of ferrous iron was less than 125 ppm. No upper iron level was determined. Because of the tremendous weight ratio of iron oxidized to carbon fixed, very large mounts of ferrous salts can be used. The optimum level of magnesium sulfate was between 0.01 and 0.1 gram per liter. The amount of ammonium sulfate provided considerably more nitrogen than was required for growth. In fact, special methods were required to demonstrate nitrogen deficiencies even when no nitrogen salt was added to the medium. Also, since ammonia and iron were the only oxidizable components of the medium, it was deemed advisable to investigate the possibility of a relation between ammonia level and the extent of iron oxidation. When ammonium sulfate was omitted entirely, the culture could be transferred four times before iron oxidation was reduced in the solution medium containing 2,000 ppm ferrous iron. Moreover, to demonstrate this effect it was necessary to keep the flasks over sulfuric acid or to bubble acid-washed air through the culture solution. Such cultures regained the ability to oxidize iron when NaNO3 was added, and could be maintained on a nitrate medium, but the rate of oxidation was slower than when ammonia was the nitrogen source. To remove the possibility that the medium contained organic materials that supported growth, in several of the foregoing experiments the flasks were cleaned with hot concentrated sulfuric acid and the water used was redistilled from acid permanganate.
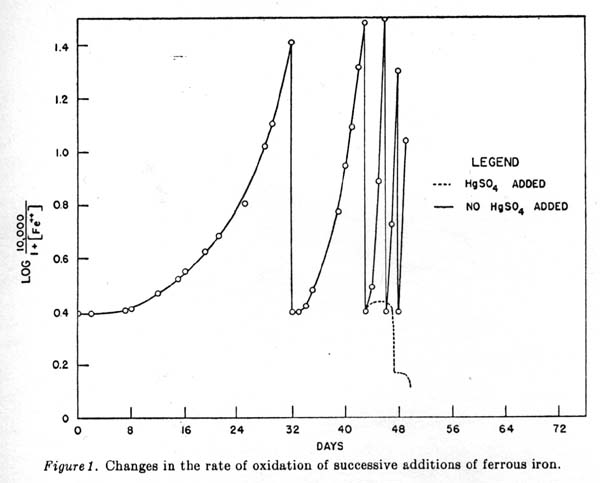
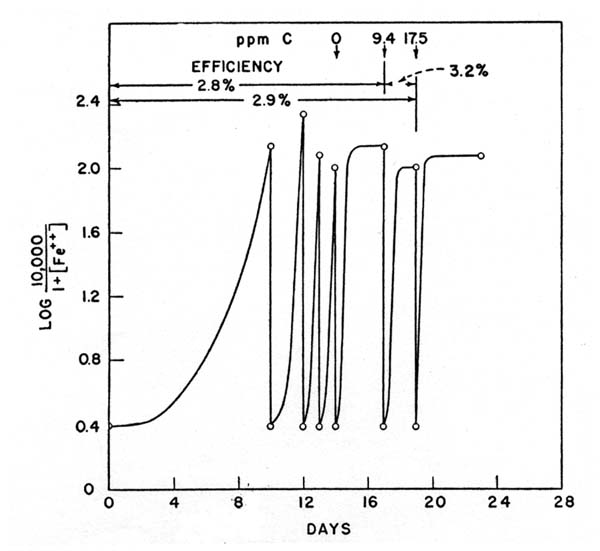
On the forty-third day the culture was divided in half and to one part 2 grams per liter of HgSO4 were added. The dotted line of figure 1 is a representation of the iron content when microbial activity ceases. No oxidation took place in the solution containing mercury, hence the successive additions of ferrous iron caused the plotted value to drop in the manner shown. Carbon fixation. For growth and iron oxidation carbon dioxide was needed and was supplied by the air. Alkali-washed air did not support either iron oxidation or cell growth. With the medium containing 2,000 ppm ferrous iron it was not possible to detect any increase in the carbon content after iron oxidation was complete. In another experiment, by adding successive amounts of ferrous iron as just described, a measurable increase in carbon was noted (figure 2).
Proof of the biological nature of oxidation. Sterile solutions of 2,000 ppm ferrous iron at pH 2.0 to 2.5 did not oxidize in the atmosphere. Sterilizing with antibacterial agents was as effective as autoclaving or filtering. Sterile solutions were bubbled with filtered air indefinitely without appreciable loss of ferrous iron. When NaN3 or iodoacetic acid was added to the media to make a concentration of 3 X 10-3 M, there was no oxidation of ferrous iron. Mercuric sulfate added to the actively oxidizing solution stopped the reaction. Cell-free filtrates of the culture solutions had no effect on the stability of ferrous solutions.
The results show conclusively that the bacterium repeatedly isolated by us from natural acid iron-rich waters continues to grow in serial transfers on a simple synthetic medium high in iron and that the oxidation of ferrous iron in this medium is dependent upon bacterial growth. The unique cultural condition that distinguishes these experiments on iron oxidation from previous ones is the high acidity at which the bacterium grows. At this pH the ferrous iron is not oxidized by atmospheric oxygen. The recent report by Gleen (1950) involving soil at pH 3.0 is the only other case of iron oxidation in an acid environment that has come to our attention. Criticisms of the claims of autotrophy of iron bacteria have called attention to the lack of conclusive evidence and to the extremely large amounts of iron that would have to be oxidized to provide energy for bacterial development. The 500:1 weight ratio of iron oxidized to cellular material produced, predicted by Starkey (1945), is consistent with the results here reported. Evidence of carbon fixation could not be obtained until extremely large amounts of iron had been oxidized, large amounts, that is, from the viewpoint of the normal iron content of natural waters. In calculating the energy utilization efficiency, the values for free energy of formation of ferrous and ferric iron were taken (Bichowsky and Rossini, 1936) instead of the values used by other authors for the reaction involving the oxidation of ferrous carbonate. Low partial pressure of carbon dioxide and high acidity (pH 2.0 to 2.5) preclude the existence of ferrous carbonate in any quantity. The efficiency value of 3.2 per cent compares with reported values of about 5 to 10 per cent for the other autotrophs (Baas-Becking and Parks, 1927). Other possibilities which might explain some of the experimental results are that the bacterium obtains its energy from the oxidation of ammonia, or traces of organic matter, or that the oxidation of ferrous iron depends upon a secondary reaction with some products of bacterial growth. The acid environment does not suggest ammonia utilization for energy. The bacterium utilizes ammonia nitrogen better than nitrate, but the concentration of ammonia has no effect upon the rate of oxidation or the total amount of iron oxidized. Moreover, the experiments with successive increments of iron rule out the possibility of ammonia oxidation serving as a main source of energy. Increases in cell numbers and in total carbon depended only on the amount of iron oxidized. When the total energy theoretically available from the complete oxidation of ammonia to nitrate is calculated, and the amount of carbon that could be fixed thereby is ascertained, the result is 0.033 grams carbon per liter. The energy utilization would probably not be over 10 per cent which would reduce the figure to 0.0033 grams C per liter, a value considerably below that obtained. The possibility of traces of organic matter serving for growth was excluded by the use of reagent grade chemicals, scrupulously clean flasks, and permanganate distilled water. The following reaction might conceivably occur in a medium containing nitrate or where all the ammoniacal nitrogen became oxidized to nitrate in some way:
It is obvious that the composition of the media as given does not include all of the elements essential to life and metabolic activity. Presumably additional essential minerals were supplied as impurities in the reagent grade chemicals used. It was impossible to provide more than traces of soluble phosphate due to the insolubility of iron phosphate. In view of the foregoing facts which show the mutual interdependence of bacterial growth and iron oxidation, the fixation of carbon during iron oxidation, and an efficiency of energy utilization consistent with theoretical considerations, it is concluded that the bacterium is an autotrophic iron oxidizer. It has already been shown that the iron-oxidizing bacterium develops autotrophically on thiosulfate (Colmer, Temple, and Hinkle, 1949). Morphologically there is no resemblance between this iron oxidizer and any of the sheathed or stalked iron bacteria. The iron bacterium cannot be distinguished from Thiobacillus thiooxidans under the microscope in stained or unstained preparations. The two organisms both produce acid in thiosulfate broth and are similar in their acid tolerance. As a gram-negative nonspore-forming motile rod living autotrophically on thiosulfate, the iron bacterium would fall into the genus Thiobacillus. It is sharply differentiated from T. thiooxidans (the member of that genus which it most resembles) by the oxidation of ferrous iron and by its failure to grow on elemental sulfur. It appears to be an obligate autotroph, failing to utilize glucose, peptone, or organic iron compounds. The ferrous iron oxidation is an outstanding characteristic of the bacterium; hence the name Thiobacillus ferrooxidans n. sp. is proposed for this organism. The iron bacterium described
in this report furnishes a clear cut example of an iron-oxidizing autotroph
according to the concept so clearly restated by Winogradsky in 1922. In
view of this fact the possibility of a mechanism for utilizing the energy
of iron oxidation must not be denied in the case of other iron bacteria
without conclusive evidence. Mere statements of the improbability of such
a mechanism are now unsound. On the other hand the present work provides
no evidence in favor of such a mechanism for any bacteria other than the
one studied, and this bacterium seems to be more closely related to the
genus Thiobacillus than to any of the previously described iron
bacteria.
An iron-oxidizing bacterium from
acid mine water has been shown to live autotrophically upon inorganic media
containing ferrous iron under conditions such that atmospheric oxidation
was excluded. Iron oxidation and cell growth are mutually interdependent,
and iron oxidation results in a measurable increase in cellular carbon.
The efficiency of the utilization of the energy available from iron oxidation
for cellular growth was found to be similar to that of other autotrophs.
The weight ratio of iron oxidized to cellular material elaborated conforms
to the requirements as stated by Starkey (1945). The bacterium, which also
grows autotrophically on thiosulfate, has been assigned to the genus Thiobacillus
and the specific name Thiobacillus ferrooxidans n. sp. is suggested.
* This work was supported by a fellowship of Bituminous Coal Research, Inc. ** Present address: Louisiana
State University, Baton Rouge, Louisiana.
BlCH0WSKY, F. R., AND ROSSINI, F. D. 1936 The thermochemistry of the chemical substances. Reinhold Publishing Corp., New York. Refer to p. 89. CATALDI, M. S. 1939 Estudio fisiologico y sistematico de algunas Chlamydobacteriales. Thesis, University of Buenos Aires, 96 pp. CHOLODNY, N. 1926 Die Eisenbakterien. Gustsv Fischer, Jena, 69-133. COLMER, A. It., AND HINELE, M. E. 1947 The role of microorganisms in acid mine drainage: A preliminary report. Science, 106, 253-256. COLMER, A. R., TEMPLE, K. L., AND HINKLE, M. E. 1949 An iron-oxidizing bacterium from the drainage of some bituminous coal mines. J. Bact., 69, 317-328. GLEEN, H. 1950 Biological oxidation of iron in soil. Nature, 166, 871-872. MOLISCH, H. 1910 Die Eisenbakterien. Gustav Fischer, Jena, 83 pp. PRINGSHElM, E. G. 1949a The filamentous bacteria Sphaerotilus, Leptothrix, Cladothrix and their relation to iron and manganese. Trans. Roy. Soc. (London), B, 233, 45. 482. PRINGSHElM, E. G. 1949b Iron bacteria. Biol. Revs. Cambridge Phil. Soc., 24, 200 24 STARKEY, R. L. 1945 Precipitation of ferric hydrate by iron bacteria. Science, 10 532-533. TREADWELL, F. P., AND HALL, W. T. 1942 Analytical chemistry, Vol. II. John Wile and Sons, Inc., New York, 545 547. VAN SLYKE, D. D., AND FOLCH, J. 1940 Manometric carbon determination. J. Biol. Chem., 136, 509-541. WINOGRADSKY, S. 1922 Eisenbakterien als Anorgoxydanten. Zentr. Bakt. Parasitenk. II, 57, 1-21. |